CAR T cell therapy: could a different approach make a difference in B cell depletion?1
CAR T cell therapy is a type of immunotherapy made from a patient’s own T cells2
What is CAR T cell therapy?3
Autologous CAR T cell therapy is a type of immunotherapy in which a patient’s own T cells are genetically modified to express chimeric antigen receptors (CARs) and then returned to the patient's body with a one-time infusion.*
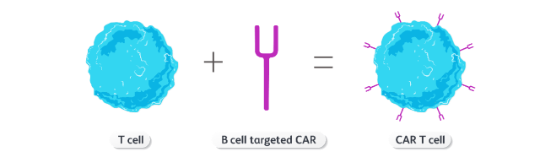
CARs confer antigen specificity, allowing CAR T cells to find and destroy specific target cells. This strategy combines the specificity of an antibody with the cytotoxic capabilities of a T cell.2,3
By targeting an antigen expressed by B cells, CAR T cells may detect and deplete autoreactive B cells that contribute to the pathogenesis and symptoms of autoimmune diseases.1,3
Following deep B cell depletion, naive B cells may emerge. It has been theorized this may reset the immune system, restoring a natural immune response.3
*Autologous CAR T treatment process includes leukapheresis, manufacturing, administration, and adverse event monitoring.4

CD19 CAR T cells are specifically engineered for deeper B cell depletion3
Learn more about the importance of B cell depletion
How CAR T cell therapy may lead to deep B cell depletion1
CAR T cell therapy is in development for the treatment of a variety of diseases3,5-8
CAR T cell therapy is being studied across a broad range of rheumatological and neuroinflammatory autoimmune diseases, including systemic lupus erythematosus (and lupus nephritis), idiopathic inflammatory myopathy, systemic sclerosis, rheumatoid arthritis, multiple sclerosis, and generalized myasthenia gravis. Early clinical research in autoimmune diseases has shown promise that supports further investigation.
Explore ongoing BMS CAR T clinical trials in autoimmune disease.5-8
Trials are open and enrolling participants.

Rheumatology (SLE [and LN])
Phase 2

Rheumatology (SLE [and LN], IIM, SSc, RA)
Phase 1

Neuroinflammatory (MS, gMG)
Phase 1
CAR=chimeric antigen receptor; gMG=generalized myasthenia gravis; IIM=idiopathic inflammatory myopathies; LN=lupus nephritis; MS=multiple sclerosis; RA=rheumatoid arthritis; SLE=systemic lupus erythematosus; SSc=systemic sclerosis.
References:
- Taubmann J, Müller F, Mutlu MY, et al. CD19 chimeric antigen receptor T cell treatment: unraveling the role of B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2024;76(4):497-504.
- Maus MV, Levine BL. Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist. 2016;21(5):608-617.
- Schett G, Mackensen A, Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023;402(10416):2034-2044.
- Beaupierre A, Lundberg R, Marrero L, Jain M, Wang T, Alencar MC. Management across settings: an ambulatory and community perspective for patients undergoing CAR T-cell therapy in multiple care settings. Clin J Oncol Nurs. 2019;23(2):27-34.
- Clinicaltrials.gov. NCT05869955. Accessed March 12, 2025.
- Data on file. REF-00068-466. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Clinicaltrials.gov. NCT06220201. Accessed March 13, 2025.
- Clinicaltrials.gov. NCT07015983. Accessed June 11, 2025.