
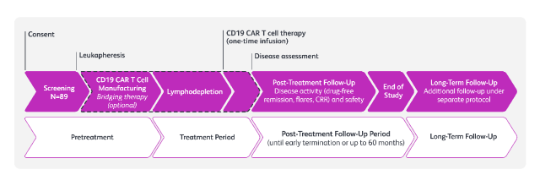
Study schema1,2

Key inclusion criteria1
Patients with an SLE diagnosis fulfilling the 2019 EULAR/ACR classification
- Active disease
- Inadequate response to glucocorticoids and ≥2 immunosuppressant therapies, used for ≥3 months

Key exclusion criteria1
Must not have other diseases, conditions, or treatments that may confound interpretation of the effects of the study drug in SLE
- Uncontrolled or clinical significant cardiovascular conditions or CNS pathology within ≥2 years
- Pregnant, nursing, or breastfeeding, or who intend to become pregnant during participation in the study
- Received live vaccines within 6 weeks
- Inadequate organ function
Please see ClinicalTrials.gov for more detailed inclusion/exclusion criteria.
Breakfree-SLE is a Phase 2, multicenter, open-label study of CC-97540 (BMS-986353), CD19-targeted NEX-T CAR T cells, in participants with active SLE (including LN) with inadequate response to glucocorticoids and ≥2 immunosuppressants1
Primary endpoint1
- Proportion of patients achieving drug-free Definition of Remission in Systemic Lupus Erythematosus (DORIS) remission at Month 6
Select secondary endpoints1
- CRR for participants with baseline LN up to Month 60
- Number of participants achieving Lupus Low Disease Activity State (LLDAS) up to Month 60
- Number of participants achieving SLE Responder Index (SRI) response (reduction of ≥4 points) with baseline SLEDAI ≥6 up to Month 60

Find a study site today
ACR=American College of Rheumatology; CAR=chimeric antigen receptor; CNS=central nervous system; CRR=complete renal response; EULAR=European Alliance of Associations for Rheumatology; LN=lupus nephritis; SLE=systemic lupus erythematosus; SLEDAI=Systemic Lupus Erythematosus Disease Activity Index.
References:
- Clinicaltrials.gov. NCT07015983. Accessed June 11, 2025.
- Data on file. REF-06869-IMM. Princeton, NJ: Bristol-Myers Squibb Company; 2025.