Aiming for more: the need for deeper B cell depletion in autoimmune diseases1,2
Are current B cell depleting strategies for the treatment of autoimmune diseases enough?2,3
Autoreactive B cells are key drivers in the pathogenesis of many autoimmune diseases. They can produce autoantibodies that target the body’s own tissues, leading to inflammation and damage. They can also act as antigen-presenting cells that activate autoreactive T cells, further driving the autoimmune response.4,5
The goal of B cell depletion is to eliminate as many autoreactive B cells as possible.5
Different strategies lead to different levels of depletion.5
Superficial B cell depletion5

Superficial B cell depletion occurs when a narrow range of B cells are targeted, or when there is ineffective tissue penetration or trafficking. Residual autoreactive B cells can cause continued disease activity and symptoms.5
Deep B cell depletion5

Deep B cell depletion aims to eliminate all autoreactive B cells, with the hope that over time, healthy naive B cells re-emerge. It has been theorized this may reset the immune system in autoimmune disease.5
A one-time infusion* of CD19 CAR T cell therapy
is being investigated for its potential to achieve deeper B cell depletion2
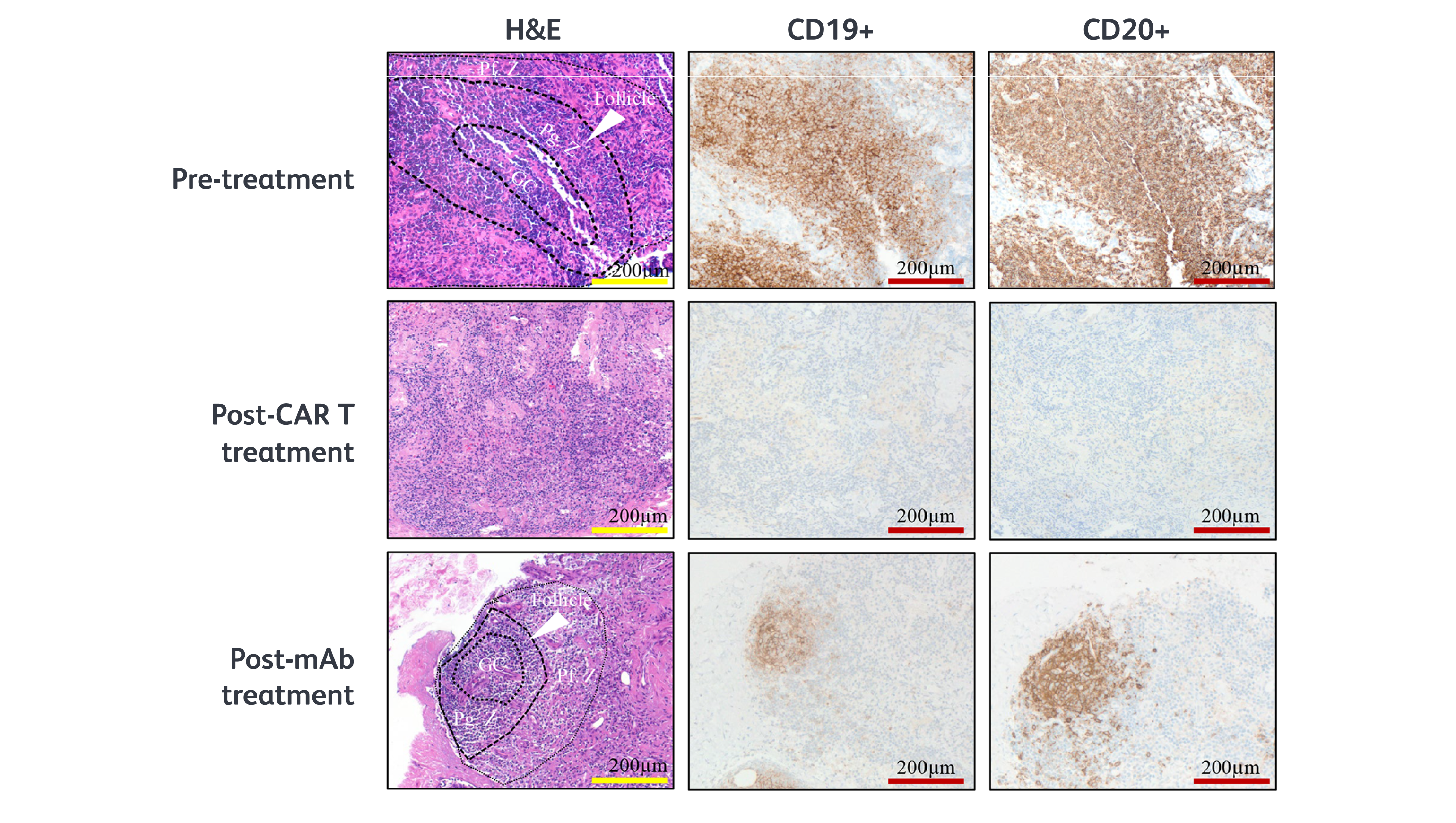
CD19 CAR T cell therapy is in development across a variety of rheumatological and neuroinflammatory autoimmune diseases. Initial studies have shown it may be able to achieve deeper B cell depletion. This strategy may allow for the detection and depletion of a wide range of B cells by combining an optimal target antigen with the migratory ability of CAR T cells. Superficial depletion may result when both conditions are not met.2,5
CD19 is an optimal target for B cell depletion
CD19 is expressed by a broad range of B cells throughout their development. Widespread expression of CD19 across B cell subtypes may allow for a more comprehensive depletion vs CD20, BAFF-R, or BCMA.3,6-8
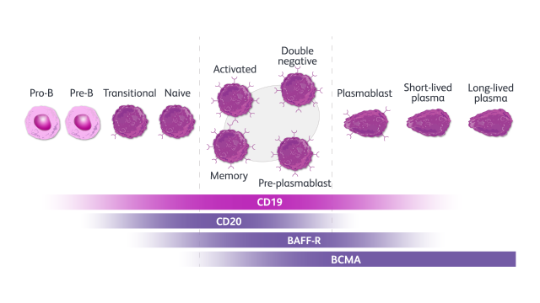
Expression of CD19, CD20, BAFF-R, and BCMA across B cell subtypes.8
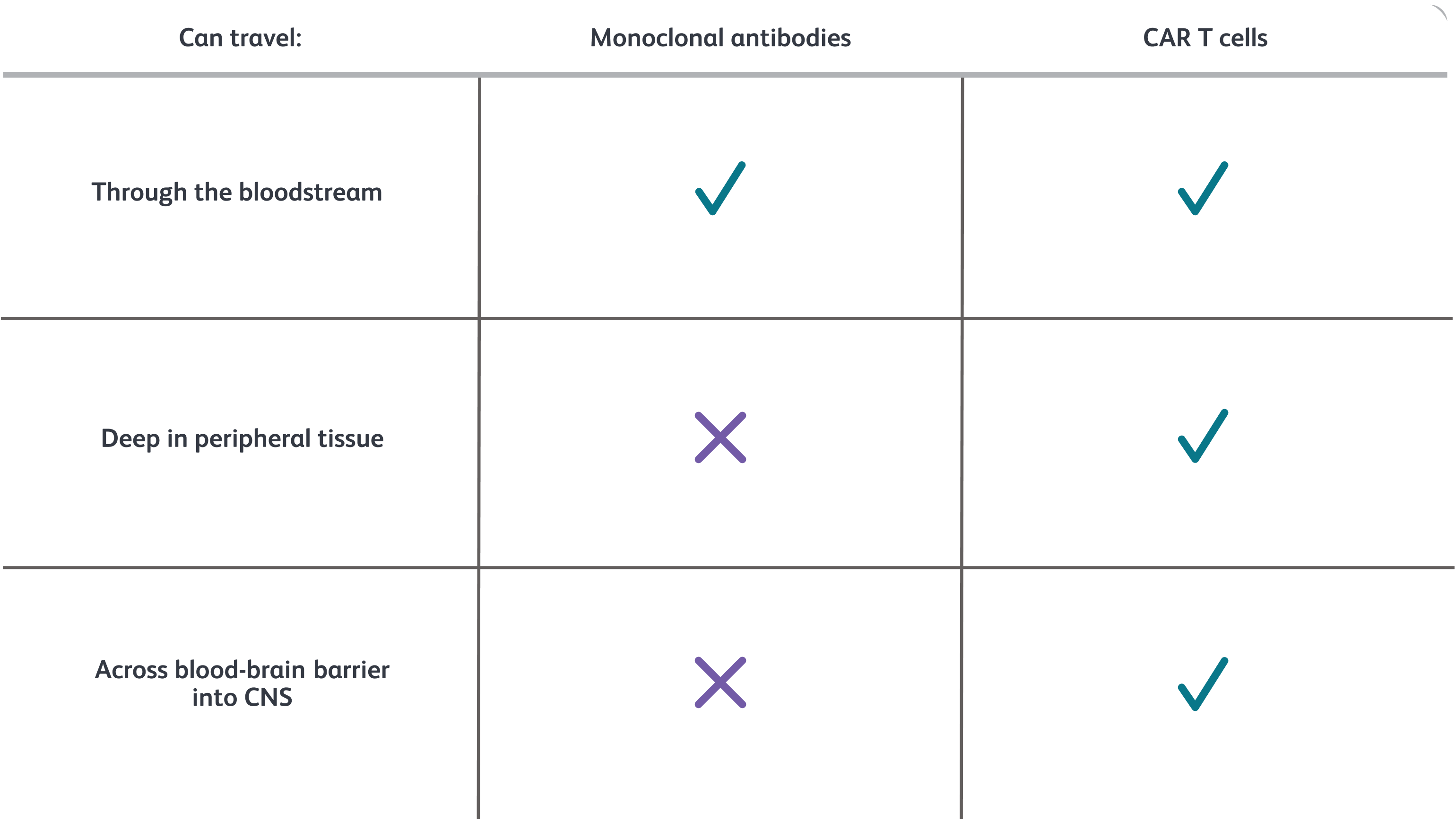
CAR T cells can travel throughout the body
These include areas where autoreactive B cells may reside but monoclonal antibodies are too large to reach. This includes deep into tissues and across the blood-brain barrier into the CNS.1,5,8-10
Explore ongoing BMS CAR T clinical trials in autoimmune disease.12-15
Trials are open and enrolling participants.

Rheumatology (SLE [and LN])
Phase 2

Rheumatology (SLE [and LN], IIM, SSc, RA)
Phase 1

Neuroinflammatory (MS, gMG)
Phase 1
*Autologous CAR T treatment process includes leukapheresis, manufacturing, administration, and adverse event monitoring16
BAFF-R=B cell activating factor receptor; BCMA=B cell maturation antigen; CAR=chimeric antigen receptor; CNS=central nervous system; gMG=generalized myasthenia gravis; H&E=hematoxylin and eosin; IIM=idiopathic inflammatory myopathies; LN=lupus nephritis; mAb=monoclonal antibody; MS=multiple sclerosis; RA=rheumatoid arthritis; SLE=systemic lupus erythematosus; SSc=systemic sclerosis.
References:
- Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-cell therapy in autoimmune disease – a case series with follow-up. N Engl J Med. 2024;390(8):687-700.
- Taubmann J, Müller F, Mutlu MY, et al. CD19 chimeric antigen receptor T cell treatment: unraveling the role of B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2024;76(4):497-504.
- Schett G, Mackensen A, Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023;402(10416):2034-2044.
- Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20(3):179-199.
- Schett G, Nagy G, Krönke G, Mielenz D. B-cell depletion in autoimmune diseases. Ann Rheum Dis. 2024;83(11):1409-1420.
- Schrezenmeier E, Janye D, Dörner T. Targeting B cells and plasma cells in glomerular diseases: translational perspectives. J Am Soc Nephrol. 2018;29(3):741-758.
- Smulski CR, Kury P, Seidel LM, et al. BAFF- and TACI-dependent processing of BAFFR by ADAM proteases regulates the survival of B cells. Cell Rep. 2017;18(9):2189-2202.
- Junt T, Calzascia T, Traggiai E, et al. Defining immune reset: achieving sustained remission in autoimmune diseases. Nat Rev Immunol. 2025. doi: 10.1038/s41577-025-01141-w.
- Kanatas P, Stouras I, Stefanis L, Stathopoulos P. B-cell-directed therapies: a new era in multiple sclerosis treatment. Can J Neurol Sci. 2023;50(3):355-364.
- Gupta S, Simic M, Sagan SA, et al. CAR-T cell-mediated B-cell depletion in central nervous system autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2023;10(2):e200080.
- Tur C, Eckstein M, Velden J, et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann Rheum Dis. 2025;84(1):106-114.
- Clinicaltrials.gov. NCT05869955. Accessed March 12, 2025.
- Data on file. REF-00068-466. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Clinicaltrials.gov. NCT06220201. Accessed March 13, 2025.
- Clinicaltrials.gov. NCT07015983. Accessed June 11, 2025.
- Beaupierre A, Lundberg R, Marrero L, Jain M, Wang T, Alencar MC. Management across settings: an ambulatory and community perspective for patients undergoing CAR T-cell therapy in multiple care settings. Clin J Oncol Nurs. 2019;23(2):27-34.